Winners / Selection Rationale
Taiyo Yakuhin Co., Ltd.
Industry Background
Unique Value Proposition
The value offered by Taiyo's generic drugs is multi-faceted including low price, ease of handling and administering for medical staff, ease in use and swallowing for patients, access to well-organized relevant medical information, and stable supply.
Prices of generic drugs are generally set at 30% lower than their original drugs based on the charges allowed by the national healthcare insurance reimbursement table. This reflects the fact that generic drugs require much less R&D cost than new drug development. Low price means lower payment by patients (this benefit is especially significant for patients with chronic disease) and also means lower expenditure for the national healthcare budget. However, in order to support the low price and make it financially viable, generic drug manufacturers must run a low-cost operation. Taiyo Pharmaceutical established a low-cost business structure including medical information provision that does not depend on a large number of Medical Representatives (MRs) but leverages high utilization rate of manufacturing facilities and highly focused R&D activities. Thus, Taiyo achieves high profitability despite the low prices set for its products.
The next stumbling block to overcome is ease of handling and taking the medicines. Generic drugs become available 15 to 20 years after the date of new drug approval for the original drugs, and as a result when putting a generic drug on the market, there is room for improvement in the areas of handling and taking the medicine. For example, a pre-filled syringe, that is a syringe filled with drug solution at the factory, reduces extra steps taken by medical staff in a hospital and improves operational efficiency, and at the same time, the risk of medical malpractice can be reduced as accidents tend to occur by the loss of drug identification when a drug solution is sucked into a syringe in a hospital. Furthermore, in order for the patients to take medicine more easily, pills can be made smaller in size so that they are easy to swallow, or granulated powder medicine can be changed into syrup. Taiyo owns a technology to produce pills that quickly dissolve in the mouth even when taken without water, and it also excels in highly sophisticated production technology for pre-filled syringes.
Taiyo Pharmaceutical has also been successful in removing the fear and anxiety hospitals and medical doctors experience with regard to generic drugs. Research indicates that the major sources of concern regarding generic drug manufacturers generally held by hospitals and doctors are lack of information provided, lack of after sale services including side-effects information, lack of stable supply as a result of not keeping sufficient stock, and lower level of quality. Taiyo succeeded in resolving these issues in its own unique ways.
Unique Value Chain
Technology development
Taiyo focuses its R&D in the areas of drug product technology and manufacturing technology. For example, in terms of the number of patents applications regarding pills that dissolve in the mouth within one minute even without drinking water, Taiyo ranks fourth after three major pharmaceutical companies (Takeda, Astellas, and Eizai). Taiyo's high level of drug production technology is widely recognized, and Taiyo is often asked to be involved in product development from the early stage of drug product designing by other pharmaceutical companies that plan to outsource manufacturing.
In 2002, Taiyo relocated its research laboratory from the suburbs of Nagoya to the center of the city where access by public transportation is much more convenient. This relocation made it easier for their research staff to commute to the laboratory, and Taiyo became able to hire female laboratory technicians as part-time staff who had been staying at home as housewives, further enabling low-cost drug development.
Product development and government approval applications
Taiyo has mastered the drug application process and has received the largest number of government approvals a year in the industry since 1996. As a result, Taiyo had 458 drug items approved from their own applications, the largest number in the generic drug industry, which greatly contributes to their broad product offering.
Manufacturing
In the generic drug industry, one of the key factors for success is to reduce manufacturing cost. As Taiyo has pursued a differentiation strategy to become a one-stop shopping site for customers by offering a broad product range, its production facilities have grown to encompass various processes and equipment as the number of its approved drugs has grown. In 1993, Taiyo began building factories to satisfy the FDA's newly established Good Manufacturing Practice (GMP) regulations and has continued to invest in expansion and improvement of manufacturing facilities over the past 12 years in order to ensure sufficient production capacity and high production quality.
Taiyo carries out various activities to reduce its manufacturing cost. First of all, it determines items and volume to be produced based on aggregated data obtained from its sales agents about actual end user purchases. Since this data is more precise in capturing actual usage than information about Taiyo's sales to its sales agents, Taiyo can avoid accumulation of unnecessary inventory. Furthermore, based on this sales data to final users, Taiyo manufactures the anticipated sales volume for a year in a single production run (as opposed to small-lot production practiced by many other generic drug manufacturers to reduce inventory cost). Although Taiyo's approach increases inventory carrying cost, it reduces total production cost and purchasing cost due to economies of scale. This approach also reduces risk of short supply, fulfilling the manufacturer's responsibility for reliable, stable product supply. Furthermore, continuous manufacturing of tablets in the same size or ampoules with the same solution volume reduces the down time for readjustment and realignment of production lines, which results in better production efficiency. In addition, Taiyo introduced the isolator system which maintains clean air in the production line by glass covers that encase only the manufacturing equipment, rather than the clean room arrangement that requires the whole production room to be isolated. As a result, running cost of the facilities as well as the burden on production staff to wear cumbersome special clean room suits is reduced. Taiyo is the largest user of such isolator systems in Japan.
Because of the smaller market size of generic drugs (about 16% of the total pharmaceutical market in Japan), utilization rate of the production capacity can become an issue. Taiyo solves this issue by performing consignment production for other pharmaceutical companies. It started consignment production as early as 1998, when "outsourcing" was unheard of in the pharmaceutical industry. Taiyo could embark on consignment production mainly because of its highly developed production technology which was well recognized in the industry. Taiyo is strong in various drug production methods that require state-of-the-art technology, including drugs produced by the freeze-dry process and drugs produced in the forms of injection solution, kit aerosol delivery system, and pre-filled syringes. Taiyo's production technology has been honed by consignment production, and its production capacity for pre-filled syringes is the third largest among all pharmaceutical companies in Japan and the largest among generic drug manufacturers.
Marketing and technical support
Taiyo Pharmaceutical emphasizes efficient provision of information to doctors and patients but does not have as many MRs as original drug manufacturers. It discloses thorough and detailed information online through its website, supplemented by its medical information call center. As a result, Taiyo needs fewer than 40 MRs to cover all of Japan.
Sales
In the generic drug industry, direct purchase and immediate one-time payment by sales agents is a common practice, but many of the independent sales agents that deal with generic drugs are relatively small (as larger wholesalers are reluctant to deal with generics because of the thinner profit margin associated with generics) and sometimes do not have the financial resources to bear inventory carrying costs.
Most of the sales agents that have distribution contracts with Taiyo Pharmaceutical are also small, with the number of employees between 3 and 60. Similar to what was traditionally done by the "door-to-door medicine vendors of Toyama" where a household was visited periodically by a vendor and given a stock of medicine for which they paid only for drugs consumed between visits, Taiyo delivers drugs to its sales agents in the volume for the anticipated sales for the coming month based on past sales data, but Taiyo does not bill the agents until they actually sell the inventory to hospitals and doctors. Taiyo has applied for a business model patent for this system which is called the pay-for-sale "inventory guaranty system." With this system to alleviate financial burden on sales agents, Taiyo has been able to establish a nationwide distribution network of 200 contracted sales agents. This network is solid with little possibility of any of the agents going out of business.
Because they incur no inventory carrying cost and can offer end users Taiyo's broad product line, most of the sales agents in the network sell only Taiyo's generic products. As a result, no internal competition with other generic drug manufacturers exists in Taiyo's distribution network.
As for other types of support to the sales agents, Taiyo provides up-to-date information on-line regarding broad topics of medicine, Taiyo's products, and healthcare administration, and also provides staff training programs for employees of sales agents.
Outbound logistics
Since Taiyo keeps the ownership of the drug inventory held on consignment by the sales agents without charging any fees, it can freely replenish the inventory. As a result, there is no need for cumbersome order placement and processing tasks. By the same token, frequency of inventory replenishing can be reduced to once or twice a month, which leads to a saving and reduction in both manpower and transportation cost.
After-sales services
Sales data Taiyo collects from its sales agents includes detailed information such as how many of each item was sold to which hospital or drug store. Should an event of unanticipated side effects occur, Taiyo can directly contact the final user immediately without depending on sales agents. (Although this is a responsibility of the pharmaceutical company required by law, it remains a source of uneasiness and anxiety for final users.)
Fit among Activities
Taking manufacturing cost reduction as an example, two approaches contribute significantly: Taiyo produces a drug in the volume anticipated to be sold in a year with a single run of the production line, and Taiyo accepts consignment manufacturing orders from other pharmaceutical companies to improve the utilization rate of its manufacturing facilities. The large production runs of the anticipated demand for a year, together with the 1-2 month inventory supply placed with sales agents, also help remove any concerns and uneasiness about possible shortage in supply.
Stock keeping at sales agents is possible because no inventory carrying cost is borne by sales agents but is instead absorbed by Taiyo. This not only contributes to stable supply but also to cost reduction in inventory management and elimination of order processing by Taiyo.
With regard to information supply, Taiyo provides a great deal of information for medical professionals on the Internet which enables dissemination of knowledge at low cost without employing a large number of MRs. Taiyo gains detailed sales information including what drug was sold to which hospitals and doctors as part of its inventory management data, which enables Taiyo to track the users of a particular drug and to directly send alerts and communicate with them in case of newly-found side effects.
As for the last item, quality, Taiyo possesses technologies for difficult pharmaceutical manufacturing processes such as injection solution. Taiyo also actively invests in manufacturing facilities that meet global standards in order to improve manufacturing quality as well as to attract production consignment outsourced by other pharmaceutical companies, which leads to a high rate of manufacturing capacity utilization and low manufacturing cost.
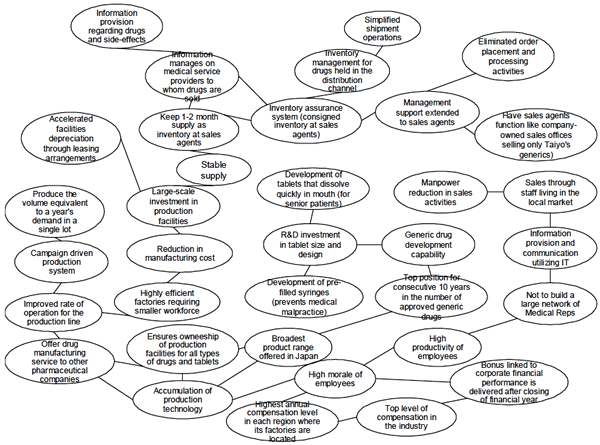
Taiyo's R&D investment is focused on seeking the best tablet design and size for both easy swallowing and handling which helps to enhance their product features and promote acceptance of the shift to generic drugs. This is in line with customer needs in the Japanese generic drug market which is still in the groundbreaking stage. (Please refer to "Activity System Map" attached for more detailed accounts of relationship among these activities.)
Innovations that Enabled Strategy
- In order to appoint financially weak small to medium drug wholesalers as Taiyo's sales agents, it arranged a system of not billing for the delivered drugs until they are sold to hospitals and pharmacists (pay-for-sale "inventory guaranty system").
- Taiyo produces total anticipated sales volume for the year in one batch, and sequentially produces tablets of the similar form and size for maximum production efficiency.
Consistency of Strategy Over Time
In 1993, Taiyo made clear its strategy of specializing in generic drugs. Various capabilities the company had developed and enhanced through new drug development were also important in obtaining government approval for generic drugs. Leveraging these accumulated capabilities, Taiyo has been able to keep the top position in number of approved drugs for a period of close to a decade from 1996 through mid-2005.
After its decision to specialize in generic drugs, Taiyo focused on developing capability for efficient manufacturing at low cost, building its distribution network and creating an excellent system for information provision. For example, in the area of information provision, Taiyo was the first generic drug manufacturer to provide a portable computer to every MR, enabling them to visit sales agents or hospitals late in the day and go home from there after work without returning to the office to file reports. In 1993, Taiyo further moved ahead and closed down and sold off seven sales offices located in major cities all over Japan, shifting to a SOHO system where MRs were able to work out of their homes instead of commuting to sales offices. Supported by this system, Taiyo currently covers all of Japan with only 40 MRs.
Trade-offs
- Taiyo does not carry out traditional methods of information provision and sales promotion activities that require many MRs.
- Taiyo does not develop new drugs but concentrates their R&D on designing tablets for ease of swallowing and handling.
- In 2002, Taiyo closed its central research laboratory in the suburbs of Nagoya and relocated it to center of the city. This move was to attract and hire female research staff as part-time employees by offering easier access and commuting.
Profitability
Return on invested capital (ROIC) (Unit = percentage point)
| Difference from industry averag over 5 year period |
Difference from industry average, by year | ||||
| 2000 | 2001 | 2002 | 2003 | 2004 | |
| 13%P | -1%P | 5.4%P | 14.2%P | 29.8%P | 8.3%P |
Return on sales (ROS) (Unit = percentage point)
| Difference from industry average over 5 year period |
Difference from industry average, by year | ||||
| 2000 | 2001 | 2002 | 2003 | 2004 | |
| 16.5%P | 13.9%P | 13.2%P | 17.2%P | 19.4%P | 15.6%P |
Activity System Map
Winners PDF
- 2005 Porter Prize Winners PDF (All of the award company in this year are published. )






